Cardiovascular Tissue Regeneration
Within the theme Cardiovascular Tissue Regeneration, we focus on advanced heart failure and associated complications, including failing blood vessels and kidney disease.
Heart disease is the #1 cause of death and accounts for an astounding 31% of all deaths world-wide. It’s closely linked with kidney disease, which affects 10% of the population. Disease of one organ often leads to malfunction of the other. It’s shocking that more pre-mature deaths occur today from non-communicable diseases like heart and kidney diseases then from easily transmitted diseases like malaria and the flu. For end-stage patients, an organ transplant is the only cure, and the shortage of organ donors is a universal problem. Heart disease is often caused by a loss of heart muscle cells, usually because of a heart attack or progressing heart failure. Current therapies, such as lifestyle changes, modern drugs, implanted devices only alleviate symptoms and prevent the disease from becoming worse. A kidney is able to repair itself after acute damage, to a state of normal functioning. However, if damage is severe, or if a patient has other underlying kidney abnormalities, this repair process fails and may lead to fibrosis and end-stage chronic kidney disease. Current treatment for end-stage kidney disease includes dialysis and transplant. There is an obvious need for solutions to reverse or repair damaged heart muscle and kidney tubules. In Utrecht, we’re drawing upon our complementary strengths by merging technology and biology to better understand these diseases. We’re focused on harnessing our natural potential for regeneration, on creating 3D models to study this potential and designing bioengineered tissues for implant.
For a better view of our work have a look on a few examples following below.
Sifting through the noise in pre-clinical data uitklapper, klik om te openen
Clinical trials for heart disease and heart regeneration are highly variable. We believe that we can better predict response to therapy if we look back into the pre-clinical metadata. In other words, data generated during the research and animal model phases. Within all the noise, we can distinguish differences between animal models, identify high-quality studies and find patterns that may lend to the design of more efficient clinical trials. And indeed, we’ve found factors that influence therapies that have been administered and can see that in large animal models, we can predict what to expect from a new drug. This will enable us to optimize and standardize models, and through computer simulations on data sets, we’ll be able to see the efficacy of each pre-clinical research approach.
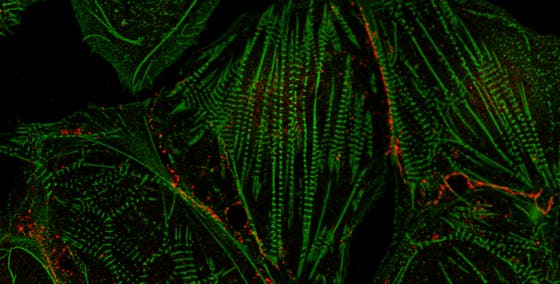
Master of heart repair uitklapper, klik om te openen
Although our heart has some regenerative capacity, it’s not enough to fully restore function, and after trauma or disease-induced damage, cardiomyocytes (heart muscle cells) start to die and scarring occurs. We believe that we’ve found the master regulator of fibrosis and we’ve seen that by silencing this gene, we can actually reverse heart fibrosis in a mouse heart. In addition, we’re looking for new biomarkers of regeneration and have observed that the stem cell gene called LGR5 can help cells become cardiomyocytes after an infarct. If we can stop or slow fibrosis, while enhancing maturation of cardiomyocytes, we may be a step further to helping our heart repair themselves
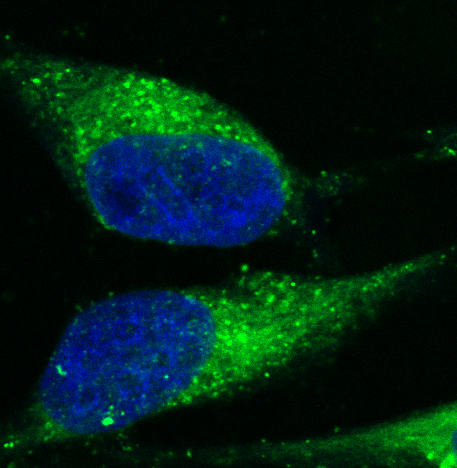
Micro heart-tissue patches uitklapper, klik om te openen
Genetics, such as a mutated gene, can influence our risk for heart disease. If the mutation is heritable, it can be passed down to family members, and if undetected or untreated, can lead to heart failure or sudden cardiac arrest. We’re studying the phospholamban (PLN) R4del mutation, which results in an enlarged heart (dilated and arrhythmogenic cardiomyopathies) and has has a high chance of sudden cardiac death. We work closely with the PLN Patient Foundation (named for this defective gene) to study this disease and to try develop a new therapy. We’ve generated induced pluripotent stem cells from PLN patients and differentiated them into heart muscle cells (cardiomyocytes). With advanced bioengineering and cell culture methods, we can mature these cells to more closely mimic the adult cardiomyocytes of the human heart, including their ability to spontaneously beat. Our next step is to screen for novel drugs that may be potential therapeutic candidates for PLN patients. We envision that this new technique can serve as a tool to study other genetic heart diseases.

Zebrafish and kidney disease uitklapper, klik om te openen

The genetic cause behind kidney disease in children is often complicated and genotype-phenotype correlations are poorly understood. Our clinical geneticists and nephrologists see families who present with many different types of kidney disease, both inherited and de novo (spontaneously occurring). We’ve conducted exome sequencing (sequencing of all expressed genes in the genome) on these patients in order to identify new genetic drivers of kidney disease. We use a zebrafish model, which has been instrumental in advancing our understanding of developmental and genetic processes. This robust fish is inexpensive, produces hundreds of offspring within weeks and grows extremely fast. Already, within the first days of development the zebrafish embryo has formed the basic functional unit of a kidney, the nephron. We’re using CRISPR/Cas9 genome editing technology to generate zebrafish that carry these mutations in order to better understand the genetics behind congenital kidney disease
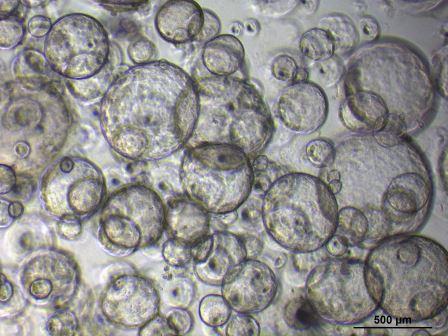
Bioengineering kidney tubules uitklapper, klik om te openen
We’re developing implantable bioengineered kidney tubules for patients with kidney disease. The proximal tubule is critical for healthy kidney function. It filters approximately two-thirds of all water and salts, and reabsorbs sugars and other essential nutrients. Both acute and chronic kidney disease usually manifest through the dysfunction of the proximal tubule. In a twostep approach, we’re defining biomaterials suitable for optimizing the extracellular matrix (ECM) layer, which is essential for structure and biochemical support of many cell types. Abnormal accumulation of the ECM leads to fibrosis, a hallmark of chronic kidney disease. We’re also culturing mini-kidney organs (organoids) from patient iPS cells, and combining these with a hollow fibrous membrane. We believe that this 3D bioengineered kidney tubule, when transplanted, will help replace normal kidney function.
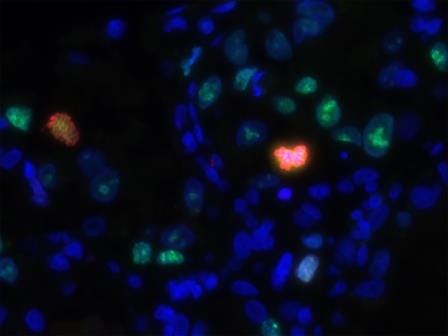
Kidney organoids uitklapper, klik om te openen
Amazingly, there are about 1 million nephrons in each kidney. These tubular structures are the functional units of the kidney and actively remove waste. After kidney injury, tubule cell death is normally followed by regeneration, however, for kidney patients this ability is insufficient. We’re using kidney organoids from human kidney biopsies or urine to study renal stem cells in kidney development, maintenance and regeneration. Moreover, organoids can be used to expand renal cells for the development of bioartificial kidneys. Interestingly, we have found that progressive renal failure is associated with an increased number of cells that can no longer divide. We’re trying to understand how to help cells make the choice to start proliferating again, or otherwise to die, in order to coax a more effective kidney regeneration process.

