Nov 12: Vulnerable elderly wanted to participate in BCG vaccine study
Nov 12: Vulnerable elderly wanted to participate in BCG vaccine study
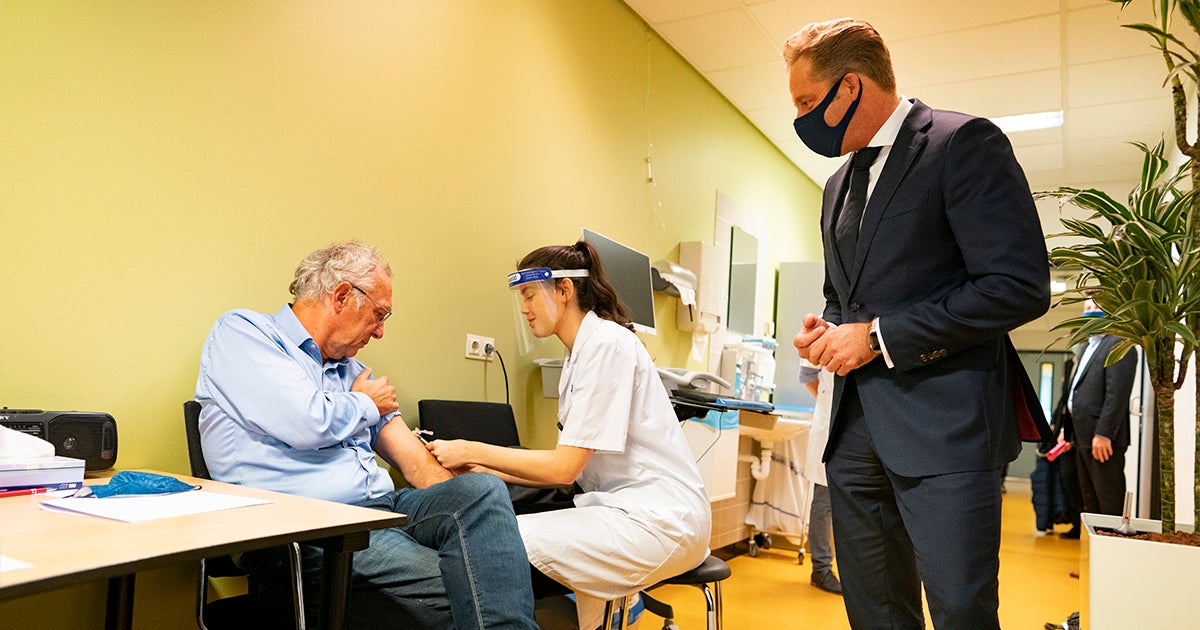
At the end of this week, 4000 participants will be participating in the Dutch study coordinated by UMC Utrecht and Radboudumc into the effect of the BCG vaccine against the consequences of a corona infection in vulnerable elderly. This was announced today during a working visit by minister Hugo de Jonge of VWS to the UMC Utrecht. The minister was provided an explanation of the ongoing investigation in which volunteers can still participate.
The coronavirus COVID-19 mainly affects vulnerable elderly people and they are most at risk of dying from the infection. Previous studies have shown that the BCG vaccine protects against tuberculosis and can increase the resistance against other infections by giving the immune system a temporary ‘boost’. This ‘boost’ may also offer protection against infection with SARS-COV-2 and other viruses.
Vulnerable elderly
For the study, vulnerable patients aged ≥60 years are approached to participate through the hospital where they are receiving treatment. The study will look at (a) the number of corona infections that occur, or (b) the occurrence of respiratory infections (including COVID-19) that require medical treatment. Until the end of this year, 20 Dutch hospitals are scheduled to enroll between 5,200 and 7,000 frail elderly, who will be followed for 6 months after vaccination. The first results are expected in the first quarter of 2021.
Recent BCG studies
The BCG-PRIME study is the fourth study in the Netherlands with the BCG vaccine against COVID-19 in a short period. Earlier this year, under the leadership of UMC Utrecht (prof. Marc Bonten) and Radboudumc (prof. Mihai Netea), three studies were already started (two in healthcare workers and one in relatively healthy elderly people) into the protective effect of the BCG vaccine against COVID 19. Worldwide there are now approximately 15 studies in which scientists are investigating whether this vaccine protects against COVID-19. In order to be able to make definitive statements about the effectiveness of the vaccine as soon as possible, it has been agreed that the data from all these studies will be analyzed in a so-called meta-analysis.
Participants wanted
Volunteers are still being sought for this placebo-controlled study. Participants are eligible if they are 60 years of age or older and are receiving treatment at a hospital, dialysis center, thrombosis service or if they have recently undergone surgery. For more information, mail to bcgprime@umcutrecht.nl.
Broad collaboration
The Ministry of Health, Welfare and Sport attaches great importance to this research and has asked ZonMw to include the study as an urgent trajectory in the research programming for COVID-19. The BCG-PRIME study was set up by UMC Utrecht with the support of the Ministry of VWS and was financed via a ZonMw grant of up to € 8.5 million. Participants in the study are the Dutch university medical centers (Amsterdam UMC, Erasmus MC, LUMC, Radboudumc, UMC Groningen, UMC Maastricht, UMC Utrecht), Santeon hospital group (Canisius-Wilhelmina Ziekenhuis, Catharina Ziekenhuis, Maasstad Ziekenhuis, Martini Ziekenhuis, Medisch Spectrum Twente, OLVG, St. Antonius Ziekenhuis) as well as Ziekenhuis Bernhoven, Haga Ziekenhuis, Ikazia Ziekenhuis, Meander Medisch Centrum, Noordwest Ziekenhuis and Rijnstate Ziekenhuis.

