Potential new strategy for cardiac regeneration through inhibition of ARID1A
Potential new strategy for cardiac regeneration through inhibition of ARID1A
Damage to the human heart, for example due to a heart attack, is not repaired and is therefore permanent. The regenerative capacity of heart muscle cells is lost soon after birth, when these cells transition to their adult form. Researchers from the Van Rooij group have identified ARID1A as an important protein in regulating this transition. In addition, they were able to stimulate cardiac regeneration by suppressing ARID1A in a model of heart damage. The results of the study were published in Nature Communications on 5 August 2023 and may contribute to the development of treatments to repair heart damage in the future.
During a heart attack, part of the heart muscle does not receive enough oxygen due to a blocked blood supply. The heart muscle cells cannot cope well with this oxygen deficiency and start to suffer damage or even die. Cardiac cells that have died cannot be regenerated in the adult heart, which means that the heart cannot recover sufficiently after a heart attack or other injury. Newborn babies' hearts, on the other hand, are better able to repair themselves by forming new heart muscle cells. Researchers from the Van Rooij group wondered how cells lose their regenerative capacity over time, and whether it would be possible to reactivate this capacity in an adult heart to repair damage.
From regeneration to maturation
Researcher Kees Boogerd explains what happens in mammalian heart muscle cells just after birth: “Initially, the heart muscle cells are still capable of recovery by dividing and thus forming new cells. We call this regeneration. After a few days, however, the cells take on their adult form. They are then optimally specialized in contraction, in order to make the heart beat firmly, but they can no longer divide properly. We call that maturation. The heart muscle cells thus switch from regeneration to maturation. We wanted to know how this switch is regulated and whether we could intervene in this process.”
ARID1A as a regulator
Through experiments in mice and lab-grown human heart tissue, the researchers discovered that the protein ARID1A plays an important role in the switch. “When we increased the amount of this protein, we saw that maturation went faster and the cells divided less. On the other hand, when we turned off ARID1A, we got the opposite effect and the cells started dividing more. The presence of ARID1A thus flips the switch in the direction of maturation: the cells mature and lose their ability to divide. We also found that ARID1A controls this by inhibiting another protein called YAP1. These discoveries give us a much better understanding of how the proliferation of heart muscle cells is regulated,” says Boogerd.
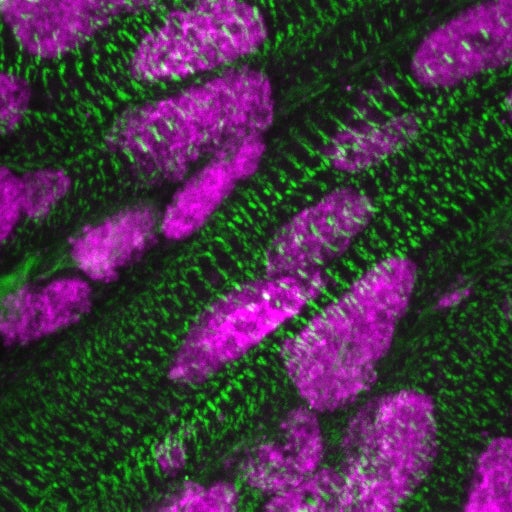
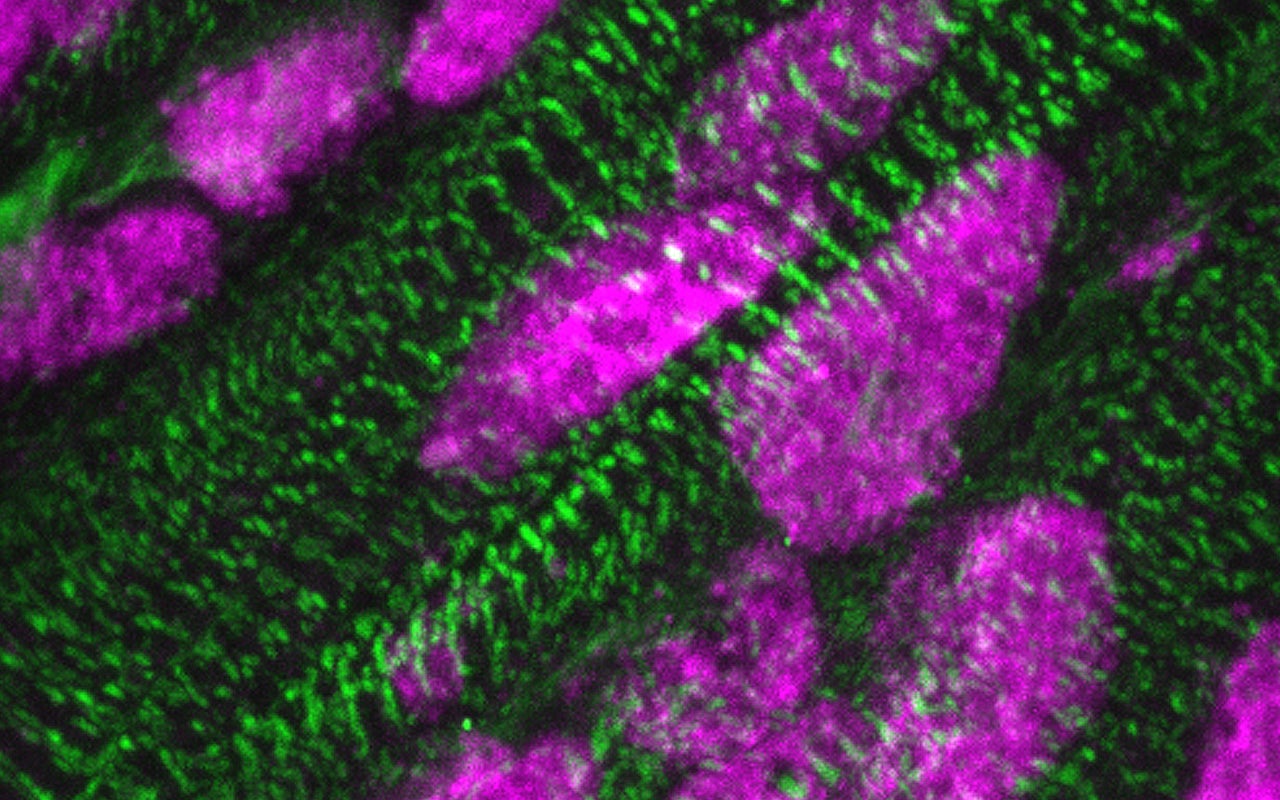
Presence of ARID1A (pink) in the nuclei of cardiac muscle cells (green), a few days after birth. Credit: Kees Boogerd. Copyright: Hubrecht Institute.
Future cardiac regeneration
With this new knowledge in hand, the researchers went one step further and investigated whether they could stimulate the repair of heart muscle cells in the event of heart damage. “When we suppressed ARID1A in our model of heart damage, we indeed saw an increase in regeneration. The heart muscle cells around the damaged tissue began to divide. That's an exciting finding, because it gives us a potential strategy to stimulate cardiac regeneration. Of course, we still need to investigate this further to better understand how we can stimulate heart tissue repair, but our results have brought us one step closer. Hopefully, this will enable us to contribute to the development of treatment options for patients with heart damage in the future,” concludes Boogerd.
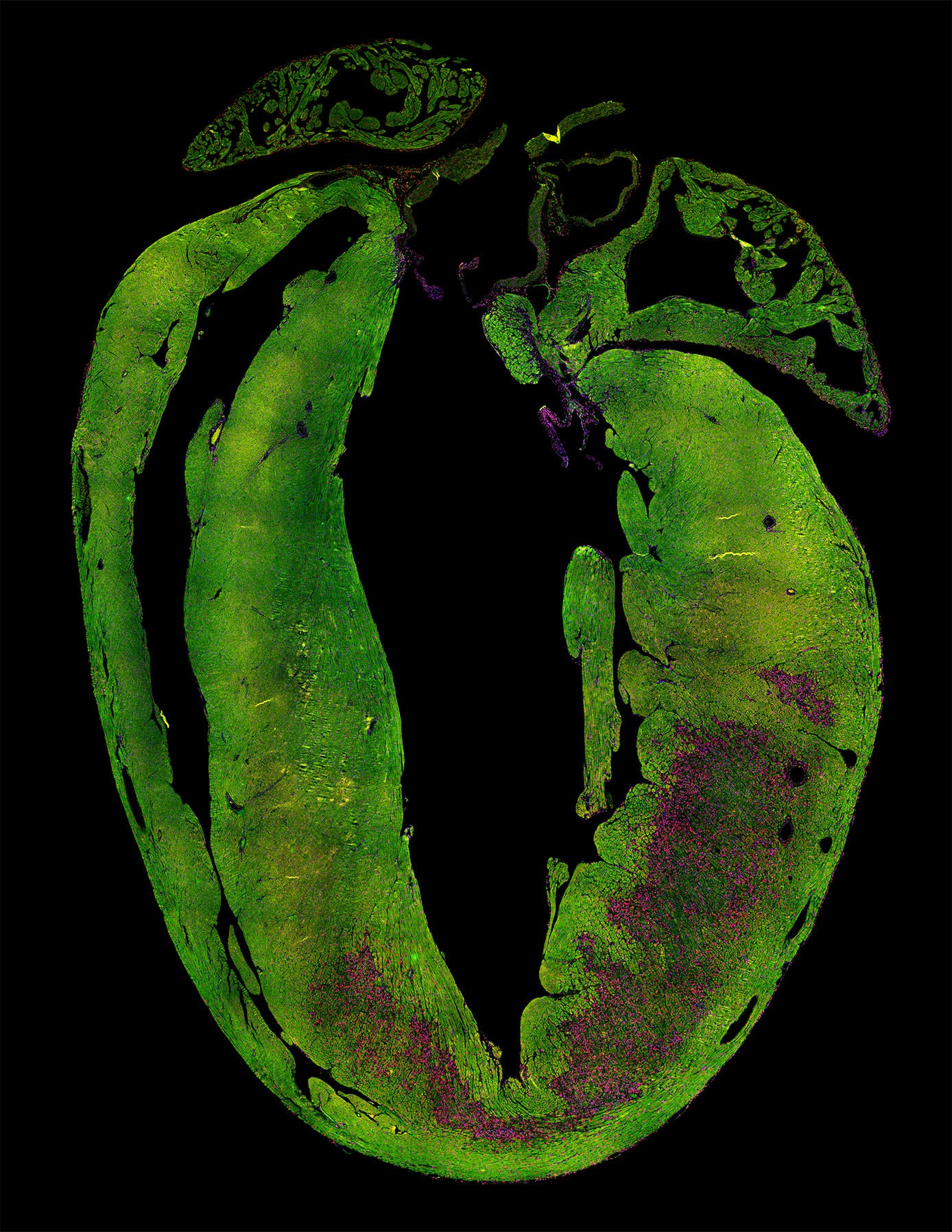
A heart attack leads to loss of heart muscle cells (green) and the formation of scar tissue (purple). This reduces the ability of the heart to contract. Credit: Kees Boogerd. Copyright: Hubrecht Institute.
Publication
Cardiomyocyte proliferation is suppressed by ARID1A-mediated YAP inhibition during cardiac maturation. Cornelis J. Boogerd, Ilaria Perini, Eirini Kyriakopoulou, Su Ji Han, Phit La, Britt van der Swaan, Jari B. Berkhout, Danielle Versteeg, Jantine Monshouwer-Kloots and Eva van Rooij. Nature Communications, 2023.

