Mutations in gene Tnnt2a cause severe cardiomyopathy
Mutations in gene Tnnt2a cause severe cardiomyopathy

Researchers from the group of Jeroen Bakkers discovered that mutations in the gene Tnnt2a cause a severe form of cardiomyopathy in zebrafish. The fish undergo structural cardiac changes, increased stress and stiffening of the wall of the heart. Additionally, they are subjected to changes in heart rhythm, contraction and calcium sensitivity. These traits are similar to those observed in human cardiomyopathies, which makes the model ideal for research into drugs for this type of disease. The results were published in the special issue on ‘Zebrafish Heart Development, Regeneration and Disease Modelling’ in the Journal of Cardiovascular Development and Disease on April 20th.
Cardiomyopathies are diseases that affect the muscles of the heart, causing sudden cardiac death and early mortality. In the human heart, there are four chambers, two ventricles and two atriums. The majority of cardiomyopathies affect the structure of the ventricles. Consequently, this can influence the structure of the atriums as well. Additionally, cardiomyopathies include traits such as a stiffened wall of the heart, dysregulation of heart rhythm and disruption in the heart’s ability to contract.
Mutational hotspot
So-called sarcomeric cardiomyopathies arise from mutations in the sarcomeres; the units responsible for heart contractions. Previous studies showed that patients suffering from sarcomeric cardiomyopathies often have mutations in the gene TNNT2. Specifically, a certain region of the gene called TNT1 is known as the mutational “hotspot” for this type of disease. There was a lack of animal models to study disease progression of mutations in TNNT2 but over the past years the zebrafish emerged as a favorable model to study heart disease. Researchers from the group of Jeroen Bakkers were therefore interested in finding out whether a mutation in the TNT1 region would result in cardiomyopathy in zebrafish.
Tnnt2a and cardiomyopathy
“We used a gene editing technique called CRISPR/Cas9 to create zebrafish with a mutation in our gene of interest. We found that zebrafish with mutations in Tnnt2a develop a severe form of cardiomyopathy during the young-adult stages of development. Sixty percent of the fish die before reaching adulthood,” Sarah Kamel, first author on the paper, explains. Zebrafish with this mutation undergo structural changes in the heart including an enlarged atrium. They also experience increased stress, stiffening of the wall of the heart, smaller ventricles and changes in heart rhythm, contraction and calcium sensitivity.
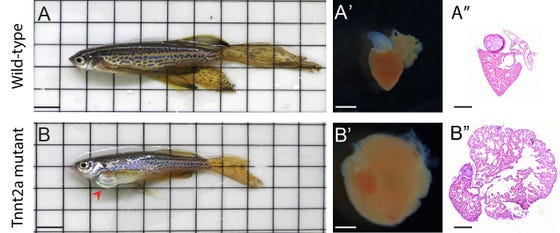
A mutation in tnnt2a causes cardiomyopathy in adult zebrafish. Credit: Sarah Kamel, copyright: Hubrecht Institute.
Potential mechanism
Tnnt2a is a sarcomeric protein that, in the presence of calcium, interacts with other components in the sarcomere to enable the heart to contract properly. “The mutation of our interest causes modifications in the TNT1 region of the gene Tnnt2a. The TNT1 region is specifically involved in the interaction with other contractile components. Furthermore, the mutation also affects the ability of calcium to propagate its signal to achieve an efficient contraction,” Kamel explains. The changes in the contractile components and the calcium propagation led to the observed changes in the heart.
Finding new drugs
Kamel: “The traits observed in the zebrafish with mutations in Tnnt2a closely resemble the traits that are present in human cardiomyopathies. This makes our model ideal for research into human cardiomyopathies and potential drugs to impact the cellular aspects this type of disease.”
Publication
A Heterozygous Mutation in Cardiac Troponin T Promotes Ca2+ Dysregulation and Adult Cardiomyopathy in Zebrafish. Sarah M. Kamel, Charlotte D. Koopman, Fabian Kruse, Sven Willekers, Sonja Chocron & Jeroen Bakkers. J. Cardiovasc. Dev. Dis. 2021.

