Biological discovery heralds new medication
Biological discovery heralds new medication

Proteins that regulate nerve cells bind to that cell's receptor to give their instructions. It was thought that when two of those proteins arrive at the same time, either one protein or the other binds. But this is not the case, according to research by UMC Utrecht and Oxford University: instead of competing, the signaling proteins both bind. This temporarily "switches off" the cell, which offers opportunities for all kinds of new therapies.
He thinks it is a great find, one that actually requires a reprint of the cell biology textbooks. Jeroen Pasterkamp, Professor of Translational Neursciences, has been researching proteins that control nerve fibers and nerve cells for some time. Jeroen: “These signaling proteins have all manner of functions. They act, for example, as a kind of traffic controller for the nerve cells, which make their way through the body during their development. They do this by binding to the receptor of a nerve cell - the landing platform as it were - and sending a signal that directs the cell in a certain direction. This creates healthy nerve pathways, but also determines, for example, how a tumor grows and spreads.”
Cell biology
Because there are quite a few of these types of signaling proteins - roughly a hundred - and because they continuously bind to nerve cells, Jeroen and his research team wondered what happens when multiple proteins arrive at the receptor at the same time. “We already knew that the proteins we are currently working with bind in the same place. The guiding principle in cell biology is that there is competition and there is a winner: either one or the other protein binds. We were particularly interested in that competition. We thought that if we had a better understanding of how that process works, we might be able to manipulate it to address a number of disease states at the molecular level.”
Large protein complex
To that end, the research team at the Oxford University laboratory first made large amounts of the receptor and ligands (the signaling proteins) and then put them together to make crystal structures. Jeroen: “To our surprise we saw that all those proteins bind to each other. Instead of competing with each other, they create a large protein complex comprising 2 ligands and 1 receptor.” They were even more astounded when they saw the biochemical result: no signal was passed on; in fact, nothing really happened anymore. “That means that the receptor effectively switches off,” says Jeroen, “and the cell becomes insensitive to the signals.” They published on this newly discovered biological principle together with colleagues from Oxford University in the journal Cell, in an article entitled: ‘Simultaneous binding of Guidance Cues NET1 and RGM blocks extracellular NEO1 signalling’
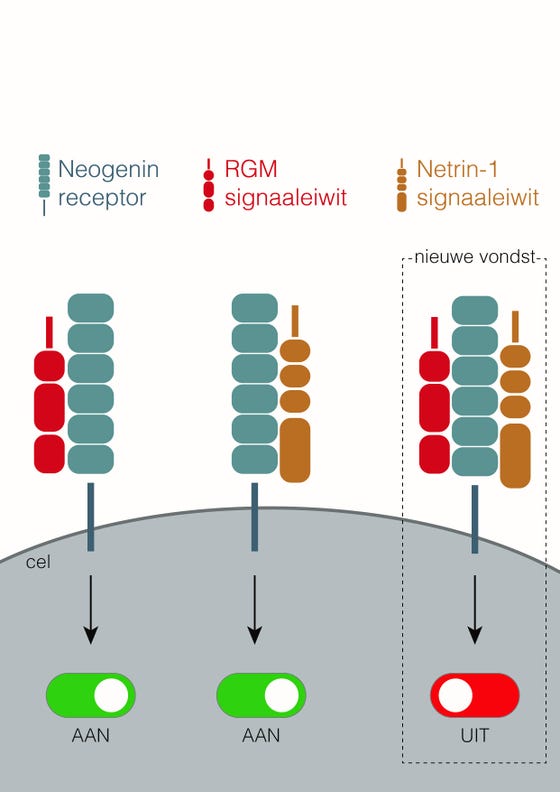
The research shows that the signaling proteins RGM and Netrin-1 do not only bind separately to the (Neogenin) receptor of a cell, but can also bind both at the same time, with the result that the receptor "switches off" and no signal is transmitted anymore.

